Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced-calorie diet and increased physical activity. Click here for full Indications and Usage.
Home / About Saxenda® / Dosing Schedule
Saxenda® Dosing Schedule
Get help staying on track with Saxenda® by connecting with your prescribing health care provider, reviewing the daily dosing schedule below, or calling the Saxenda® Hotline at 1-844-845-6913 Monday through Friday 8:30 AM to 6:00 PM ET.
Need reminders for when to take Saxenda®? Try setting an alarm on a smartphone or AI assistant (like Alexa or Siri).
Review the Saxenda® dosing schedule
The Saxenda® daily dosing schedule was designed to help patients get used to their new medicine while also minimizing gastrointestinal side effects. Review the schedule below, and be sure to schedule a 4-month follow-up with your prescribing health care provider. This visit can be sooner if there are questions or concerns about dosing or side effects. As a reminder, a Saxenda® prescription contains enough medicine for 30 days.
Below is a dosing schedule to guide you through the starting dosage of 0.6 mg to the dosage of 3 mg.

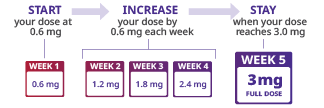
Saxenda® should be taken exactly as prescribed. After starting Saxenda®, provided there are no issues with tolerating it, the dose should be increased weekly until the 3 mg dose is reached. If you or your teen have trouble tolerating an increased dose, or if you have any other questions, be sure to call your (or your teen's) health care provider. The Saxenda® dose should only be changed if your (or your teen's) health care provider advises to do so.
Nausea is the most common side effect when first starting Saxenda®. Learn more about ways to manage it here. For additional side effects, please refer to the Medication Guide.
If you (or your teen) miss your daily dose of Saxenda®, just take the next daily dose as usual on the following day. Do not take an extra dose of Saxenda® or increase the dose on the following day to make up for the missed dose. If Saxenda® is not taken for 3 days or more, call your respective health care provider to talk about how to restart treatment.
What is Saxenda®?
Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced calorie diet and increased physical activity.
- Saxenda® and Victoza® have the same active ingredient, liraglutide, and should not be used together or with other GLP-1 receptor agonist medicines
- It is not known if Saxenda® is safe and effective when taken with other prescription, over-the-counter medicines, or herbal weight-loss products
- It is not known if Saxenda® is safe and effective in children under 12 years of age
- It is not known if Saxenda® is safe and effective in children aged 12 to 17 years with type 2 diabetes
Important Safety Information
Do not share your Saxenda® pen with others even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
What is the most important information I should know about Saxenda®?
Serious side effects may happen in people who take Saxenda®, including:
Possible thyroid tumors, including cancer. Tell your health care professional if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats and mice, Saxenda® and medicines that work like Saxenda® caused thyroid tumors, including thyroid cancer. It is not known if Saxenda® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
Do not use Saxenda® if you or any of your family have ever had MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not use Saxenda®?
Do not use Saxenda® if:
- you or any of your family have ever had MTC or if you have MEN 2
- you have had a serious allergic reaction to liraglutide or any of the ingredients in Saxenda®. See symptoms of serious allergic reactions in "What are the possible side effects of Saxenda®?"
- you are pregnant or plan to become pregnant. Saxenda® may harm your unborn baby
Before taking Saxenda®, tell your health care provider about all of your medical conditions, including if you:
- are taking certain medicines called GLP-1 receptor agonists
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food
- have or have had problems with your pancreas, kidneys or liver
- have or have had depression or suicidal thoughts, or mental health issues
- are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation)
- are breastfeeding or plan to breastfeed. It is not known if Saxenda® passes into your breast milk. You and your health care provider should decide if you will use Saxenda® or breastfeed
Tell your health care provider about all the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements. Saxenda® slows stomach emptying and can affect medicines that need to pass through the stomach quickly. Saxenda® may affect the way some medicines work and some other medicines may affect the way Saxenda® works. Tell your health care provider if you take diabetes medicines, especially insulin and sulfonylurea medicines.
How should I use Saxenda®?
- Read the Instructions for Use that comes with Saxenda®
- Inject your dose of Saxenda® under the skin (subcutaneously) in your stomach area (abdomen), upper leg (thigh), or upper arm, as instructed by your health care provider. Do not inject into a vein or muscle
- Change (rotate) your injection site within the area you choose with each injection to reduce your risk of getting lumps under the skin (cutaneous amyloidosis). Do not use the same site for each injection
What are the possible side effects of Saxenda®?
Saxenda® may cause serious side effects, including:
- inflammation of the pancreas (pancreatitis). Stop using Saxenda® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your stomach area (abdomen) to your back
- gallbladder problems. Saxenda® may cause gallbladder problems, including gallstones. Some gallbladder problems need surgery. Call your health care provider if you have any of the following symptoms: pain in your upper stomach (abdomen), fever, yellowing of your skin or eyes (jaundice), or clay-colored stools
- increased risk of low blood sugar (hypoglycemia) in adults with type 2 diabetes who also take medicines to treat type 2 diabetes such as sulfonylureas or insulin
- risk of low blood sugar (hypoglycemia) in children who are 12 years of age and older without type 2 diabetes
- Signs and symptoms of low blood sugar may include: shakiness, sweating, headache, drowsiness, weakness, dizziness, confusion, irritability, hunger, fast heartbeat, and feeling jittery. You should check your blood sugar before you start taking Saxenda® and while you take Saxenda®
- increased heart rate. Saxenda® can increase your heart rate while you are at rest. Your health care provider should check your heart rate while you take Saxenda®. Tell your health care professional if you feel your heart racing or pounding in your chest and it lasts for several minutes
- kidney problems (kidney failure). Saxenda® may cause nausea, vomiting, or diarrhea leading to loss of fluids (dehydration). Dehydration may cause kidney failure, which can lead to the need for dialysis. This can happen in people who have never had kidney problems before. Drinking plenty of fluids may reduce your chance of dehydration. Call your health care provider right away if you have nausea, vomiting, or diarrhea that does not go away, or if you cannot drink liquids by mouth
- serious allergic reactions. Stop using Saxenda® and get medical help right away if you have any symptoms of a serious allergic reaction including swelling of your face, lips, tongue, or throat, fainting or feeling dizzy, very rapid heartbeat, problems breathing or swallowing, or severe rash or itching
- depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes, in your mood, behaviors, thoughts, or feelings. Call your health care provider right away if you have any mental changes that are new, worse, or worry you
- food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Saxenda® may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Saxenda® before you are scheduled to have surgery or other procedures
The most common side effects of Saxenda® in adults include nausea, diarrhea, constipation, vomiting, injection site reaction, low blood sugar (hypoglycemia), headache, tiredness (fatigue), dizziness, stomach pain, and change in enzyme (lipase) levels in your blood. Additional common side effects in children are fever and gastroenteritis.
What is Saxenda®?
Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced calorie diet and increased physical activity.
- Saxenda® and Victoza® have the same active ingredient, liraglutide, and should not be used together or with other GLP-1 receptor agonist medicines
- It is not known if Saxenda® is safe and effective when taken with other prescription, over-the-counter medicines, or herbal weight-loss products
- It is not known if Saxenda® is safe and effective in children under 12 years of age
- It is not known if Saxenda® is safe and effective in children aged 12 to 17 years with type 2 diabetes
Please click here for Prescribing Information and Medication Guide for Saxenda®.
Saxenda® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is Saxenda®?
Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced calorie diet and increased physical activity.
- Saxenda® and Victoza® have the same active ingredient, liraglutide, and should not be used together or with other GLP-1 receptor agonist medicines
- It is not known if Saxenda® is safe and effective when taken with other prescription, over-the-counter medicines, or herbal weight-loss products
- It is not known if Saxenda® is safe and effective in children under 12 years of age
- It is not known if Saxenda® is safe and effective in children aged 12 to 17 years with type 2 diabetes
Important Safety Information
Do not share your Saxenda® pen with others even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
What is the most important information I should know about Saxenda®?
Serious side effects may happen in people who take Saxenda®, including:
Possible thyroid tumors, including cancer. Tell your health care professional if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats and mice, Saxenda® and medicines that work like Saxenda® caused thyroid tumors, including thyroid cancer. It is not known if Saxenda® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
Do not use Saxenda® if you or any of your family have ever had MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not use Saxenda®?
Do not use Saxenda® if:
- you or any of your family have ever had MTC or if you have MEN 2
- you have had a serious allergic reaction to liraglutide or any of the ingredients in Saxenda®. See symptoms of serious allergic reactions in "What are the possible side effects of Saxenda®?"
- you are pregnant or plan to become pregnant. Saxenda® may harm your unborn baby
Before taking Saxenda®, tell your health care provider about all of your medical conditions, including if you:
- are taking certain medicines called GLP-1 receptor agonists
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food
- have or have had problems with your pancreas, kidneys or liver
- have or have had depression or suicidal thoughts, or mental health issues
- are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation)
- are breastfeeding or plan to breastfeed. It is not known if Saxenda® passes into your breast milk. You and your health care provider should decide if you will use Saxenda® or breastfeed
Tell your health care provider about all the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements. Saxenda® slows stomach emptying and can affect medicines that need to pass through the stomach quickly. Saxenda® may affect the way some medicines work and some other medicines may affect the way Saxenda® works. Tell your health care provider if you take diabetes medicines, especially insulin and sulfonylurea medicines.
How should I use Saxenda®?
- Read the Instructions for Use that comes with Saxenda®
- Inject your dose of Saxenda® under the skin (subcutaneously) in your stomach area (abdomen), upper leg (thigh), or upper arm, as instructed by your health care provider. Do not inject into a vein or muscle
- Change (rotate) your injection site within the area you choose with each injection to reduce your risk of getting lumps under the skin (cutaneous amyloidosis). Do not use the same site for each injection
What are the possible side effects of Saxenda®?
Saxenda® may cause serious side effects, including:
- inflammation of the pancreas (pancreatitis). Stop using Saxenda® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your stomach area (abdomen) to your back
- gallbladder problems. Saxenda® may cause gallbladder problems, including gallstones. Some gallbladder problems need surgery. Call your health care provider if you have any of the following symptoms: pain in your upper stomach (abdomen), fever, yellowing of your skin or eyes (jaundice), or clay-colored stools
- increased risk of low blood sugar (hypoglycemia) in adults with type 2 diabetes who also take medicines to treat type 2 diabetes such as sulfonylureas or insulin
- risk of low blood sugar (hypoglycemia) in children who are 12 years of age and older without type 2 diabetes
- Signs and symptoms of low blood sugar may include: shakiness, sweating, headache, drowsiness, weakness, dizziness, confusion, irritability, hunger, fast heartbeat, and feeling jittery. You should check your blood sugar before you start taking Saxenda® and while you take Saxenda®
- increased heart rate. Saxenda® can increase your heart rate while you are at rest. Your health care provider should check your heart rate while you take Saxenda®. Tell your health care professional if you feel your heart racing or pounding in your chest and it lasts for several minutes
- kidney problems (kidney failure). Saxenda® may cause nausea, vomiting, or diarrhea leading to loss of fluids (dehydration). Dehydration may cause kidney failure, which can lead to the need for dialysis. This can happen in people who have never had kidney problems before. Drinking plenty of fluids may reduce your chance of dehydration. Call your health care provider right away if you have nausea, vomiting, or diarrhea that does not go away, or if you cannot drink liquids by mouth
- serious allergic reactions. Stop using Saxenda® and get medical help right away if you have any symptoms of a serious allergic reaction including swelling of your face, lips, tongue, or throat, fainting or feeling dizzy, very rapid heartbeat, problems breathing or swallowing, or severe rash or itching
- depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes, in your mood, behaviors, thoughts, or feelings. Call your health care provider right away if you have any mental changes that are new, worse, or worry you
- food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Saxenda® may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Saxenda® before you are scheduled to have surgery or other procedures
The most common side effects of Saxenda® in adults include nausea, diarrhea, constipation, vomiting, injection site reaction, low blood sugar (hypoglycemia), headache, tiredness (fatigue), dizziness, stomach pain, and change in enzyme (lipase) levels in your blood. Additional common side effects in children are fever and gastroenteritis.
What is Saxenda®?
Saxenda® (liraglutide) injection 3 mg is an injectable prescription medicine used for adults with excess weight (BMI ≥27) who also have weight-related medical problems or obesity (BMI ≥30), and children aged 12-17 years with a body weight above 132 pounds (60 kg) and obesity to help them lose weight and keep the weight off. Saxenda® should be used with a reduced calorie diet and increased physical activity.
- Saxenda® and Victoza® have the same active ingredient, liraglutide, and should not be used together or with other GLP-1 receptor agonist medicines
- It is not known if Saxenda® is safe and effective when taken with other prescription, over-the-counter medicines, or herbal weight-loss products
- It is not known if Saxenda® is safe and effective in children under 12 years of age
- It is not known if Saxenda® is safe and effective in children aged 12 to 17 years with type 2 diabetes
Please click here for Prescribing Information and Medication Guide for Saxenda®.
Saxenda® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
